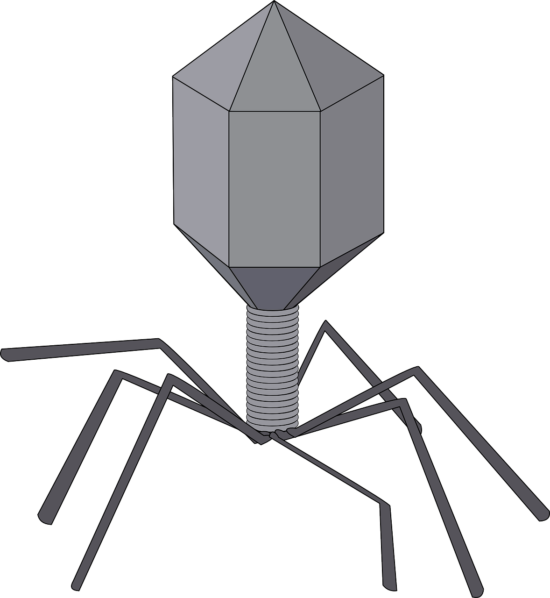
The antimicrobial potential of bacteriophages
This is a House of Commons Committee report, with recommendations to government. The Government has two months to respond.
The further development of phage therapies face significant challenges that will need to be overcome if their potential is to be fully realised. Like many personalised medicines, they currently struggle to meet regulations designed for conventional medicines produced to a single formulation. In the UK phages have not been manufactured to required Good Manufacturing Process (GMP) standards, precluding UK-produced phages from clinical use. The small quantities of phages that have been used in the UK are imported, usually not to GMP standards, and are heavily restricted because they are unlicensed.
AMR NEWS
Your Biweekly Source for Global AMR Insights!
Stay informed with the essential newsletter that brings together all the latest One Health news on antimicrobial resistance. Delivered straight to your inbox every two weeks, AMR NEWS provides a curated selection of international insights, key publications, and the latest updates in the fight against AMR.
Don’t miss out on staying ahead in the global AMR movement—subscribe now!